american academy of pediatrics covid vaccine recommendations
A 2 dose primary series is recommended. Dose 2 should be given at least 1 month 28 days after receipt of the first dose.

Kids Covid Vaccine Twitter Chat Twitter
The Advisory Committee on Immunization Practices ACIP of the Centers for Disease Control and Prevention CDC has recommended two COVID-19 vaccines.

. All available safety and efficacy data are reviewed before authorization or. Kids should eat about one to four cups of vegetables per day depending on their age sex and activity level. The nearly 4 million children diagnosed with COVID-19 so far account for 14 of cases according to a recent report by the American Academy of Pediatrics AAP and the Childrens Hospital Association CHA.
Ad FDA has authorized the Moderna COVID-19 Vaccine under an Emergency Use Authorization. The American Academy of Pediatrics AAP recommends the following related to coronavirus disease 2019 COVID-19 vaccine in children and adolescents. 1 12 to 2 cups of fruit when they are 9 to 13 years old.
By and large its a disease that spreads from adults to adults says H. The best way to slow the emergence of COVID variants is to get vaccinated. A two-dose Moderna COVID-19 vaccine series 50µg is recommended.
Learn More About A Vaccine Option On The Site. The Centers for Disease Control and Prevention CDC signed off on boosters for this age group Thursday after its Advisory Committee on Immunization Practices ACIP recommended the boosters in an 11-1 vote with one abstention. 1 12 to 2 cups girls or 2 to 2 12 cups boys of fruit when they are 14 to 18 years old 26.
COVID Vaccines for Babies Young Kids. One for children ages 6 months to 4 years and one for ages 6 months to 5 years. The doses should be given 28-days apart.
Interim Guidance Find the latest AAP guidance to help you provide optimal care for your patients and families during the COVID-19 pandemic. MRNA COVID-19 vaccines are safe and effective at the recommended intervals but a longer interval may be considered for some populations specifically males ages 12-39 years. Department of Health and Human Services they are published in CDCs Morbidity.
A single booster dose of the Pfizer vaccine is authorized for kids 5 through 17 years old at least 5 months after the second dose of vaccine. CDC guide helps pediatricians prepare for COVID vaccination of children under 5 May 18 2022 -- Both Moderna and Pfizer have adapted their COVID-19 vaccines for children under 5 years. The Committees recommendations are forwarded to CDCs Director for approval.
AAP recommend COVID-19 vaccination for all children 6 months of age and older who do not have contraindications. ACIP approved the following recommendations by majority vote at its June 24-25 2021 meeting. The ACIP develops recommendations on how to use vaccines to control disease in the United States.
ACIP recommends 3-doses of Dengvaxia administered 6 months apart at month 0 6 and 12 in persons 9-16 years of age with a laboratory confirmation of previous dengue infection and living in endemic areas. Recommendations for eating vegetables are similar. COVID-19 Vaccination Recommendations for Children.
How to Use Imported Baby Formula. One or both could be available in June. FDA authorization of the Pfizer-BioNTech COVID-19 Vaccine allows children who will turn from age 11 years to 12 years between their first and second dose in the primary series to receive for either dose.
Learn more about the facts behind the COVID-19 Vaccine. Examples of these professional organizations with which ACIP develops the annual harmonized childhood schedule are the American Academy of Pediatrics AAP and the American Academy of Family Physicians AAFP. COVID-19 vaccines available for children include.
1 the Pfizer-BioNTech COVID-19 Vaccine product authorized for children ages 511 years or 2 the Pfizer. Ad Maximize protection get all recommended vaccine doses and boosters as soon as possible. COVID Vaccines Authorized for Children Ages 6 Months Up.
The AAP recommends COVID-19 vaccination for all children and adolescents 5 years of age and older who do not have contraindications using a COVID-19 vaccine authorized for use for their age. A two-dose Moderna COVID-19 vaccine series is recommended for children ages 6 months through 5 years under the FDAs Emergency Use Authorization EUA. The American Academy of Pediatrics AAP supports this recommendation and encourages.
Questions About The Vaccine. Children who will turn from age 11 years to 12 years. FDA authorizes COVID vaccine boosters for children ages 5-11.
One for children ages 6 months through 4 years and one for ages 6 months through 5 years. Human Papillomavirus and Other Vaccines Recommended for Adolescents. ITASCA ILToday the Advisory Committee on Immunization Practices ACIP of the Centers for Disease Control and Prevention CDC recommended two COVID-19 vaccines.
Cody Meissner MD chief of the Division of Pediatric. New vaccines are evaluated by a long-standing rigorous and transparent process by the US Food and Drug Administration and the Centers for Disease Control and Prevention CDC. In addition to vaccinations the AAP recommends a layered approach to make school safe for all students teachers and staff.
CDC recommends COVID-19 vaccines for everyone ages 6 months and older and boosters for everyone ages 5 years and older if eligible. Vaccines are safe and effective in protecting individuals and populations against infectious diseases. The AAP Parenting Website.
The AAP recommends COVID-19 vaccination for all children and adolescents 12 years of age and older who do not have contraindications using a COVID-19 vaccine authorized for use for their age. The AAFP has reviewed and approved the recommendation to prevent serious illness in this age group. The viral vector vaccine in the United States is given in one dose to people 18 years and older.
Once the ACIP recommendations have been reviewed and approved by the CDC Director and the US. In updated guidance for the 2021-22 school year the American Academy of Pediatrics AAP strongly recommends in-person learning and urges all who are eligible to be vaccinated to protect against COVID-19. The AAP offers QI and education courses on providing HPV and other adolescent vaccines information about why AAP recommends HPV vaccination starting at age 9 the Same Way Same Day app and resources from the American Cancer Society.
Heres what the AAP says on kid vaccines. The American Academy of Pediatrics helped parents understand COVID with faster guidelines than the CDCs. Start the day smarter Notable deaths in.
The recommendation followed the Food and Drug Administration authorization of two long-awaited COVID-19 vaccines for children under the age of 5 years old. The American Academy of Pediatrics AAP supports this. The ACIP works with 30 professional organizations that are highly regarded in the health field.
Ages 12 17 years. Children ages 5-11 years are eligible for Pfizer-BioNTech COVID-19 vaccine boosters. The two vaccines include the Pfizer-BioNTech COVID-19 vaccine for ages 6 months to 4 years old and the Moderna COVID-19 vaccine for ages 6 months to 17 years old.
Ad Visit The Consumer Website To Learn About A Vaccine Option. Any COVID-19 vaccine authorized through Emergency Use Authorization by the US Food and Drug Administration recommended by the CDC and appropriate by age and.

Covid 19 Information And Resources Tnaap
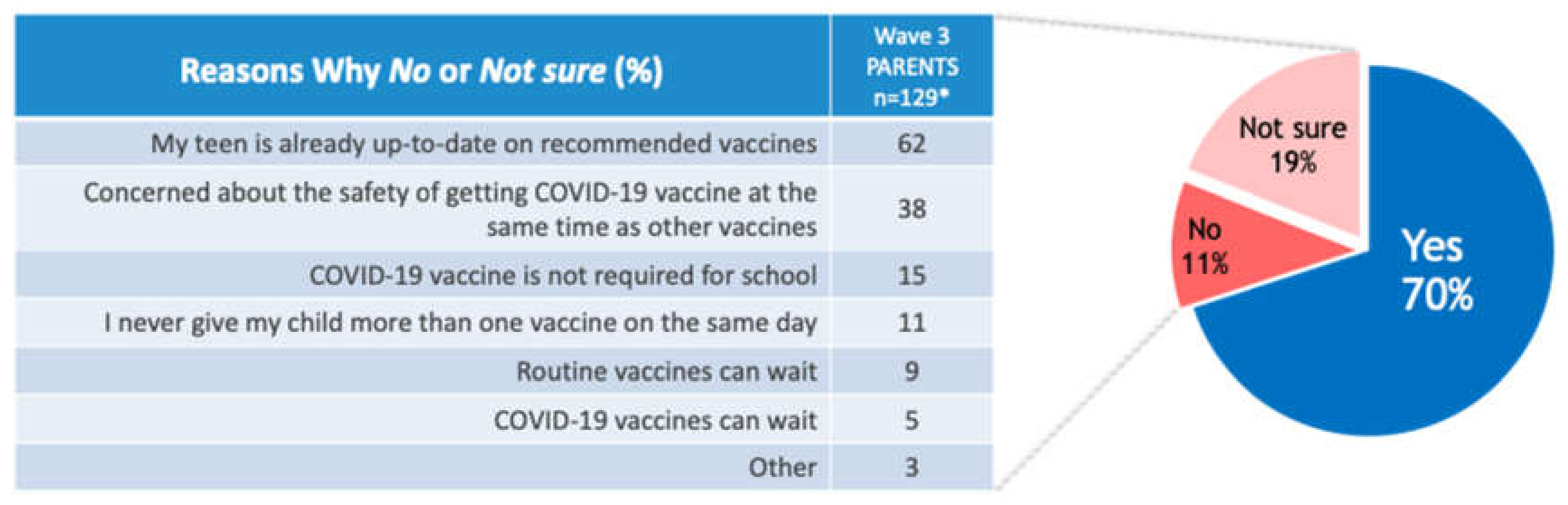
Vaccines Free Full Text Vaccine Hesitancy In The Time Of Covid 19 Attitudes And Intentions Of Teens And Parents Regarding The Covid 19 Vaccine Html

Covid 19 Information And Resources Tnaap

American Academy Of Pediatrics Pediatricians As Omicron Surges It S More Important Than Ever For Eligible Children And Teens To Get Their Covid Vaccine And Booster Shots You Can Help Families Make

American Academy Of Pediatrics Flu Season Is Here And Covid 19 Is Still Circulating Get The Flu Vaccine And Do Your Part To Protect Your Loved Ones And Your Community This Season
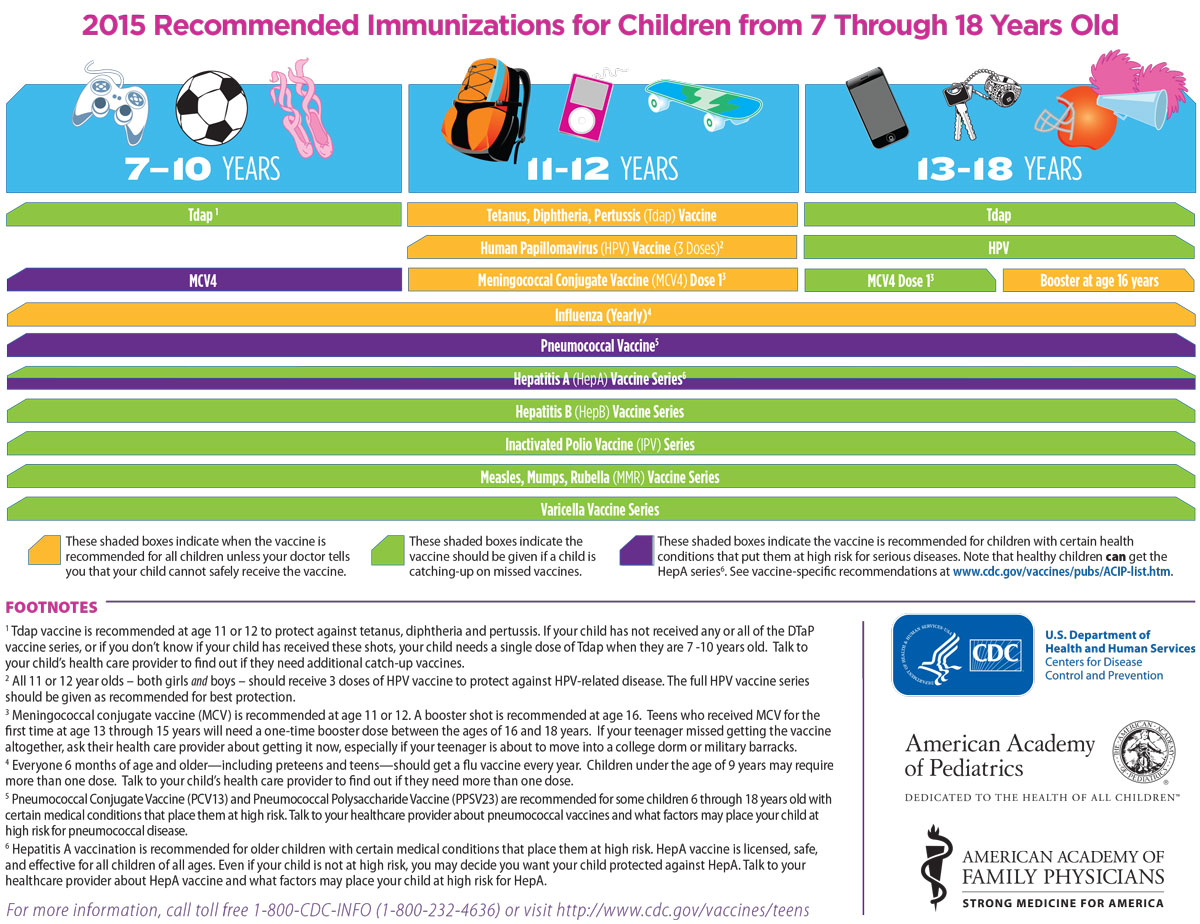
Immunization Schedule 7 18 Yrs Maryland Farms Pediatrics

Covid 19 Information And Resources Tnaap

Covid 19 Vaccine Use In Children Conversation Aafp

American Academy Of Pediatrics On Twitter It S Finally Here Covid 19 Vaccines Are Now Available For Kids Ages 6 Months To 5 Years Vaccination Is The Best Way To Protect Your Child Callyourpediatrician

Pediatric Vaccine Social Media Toolkit
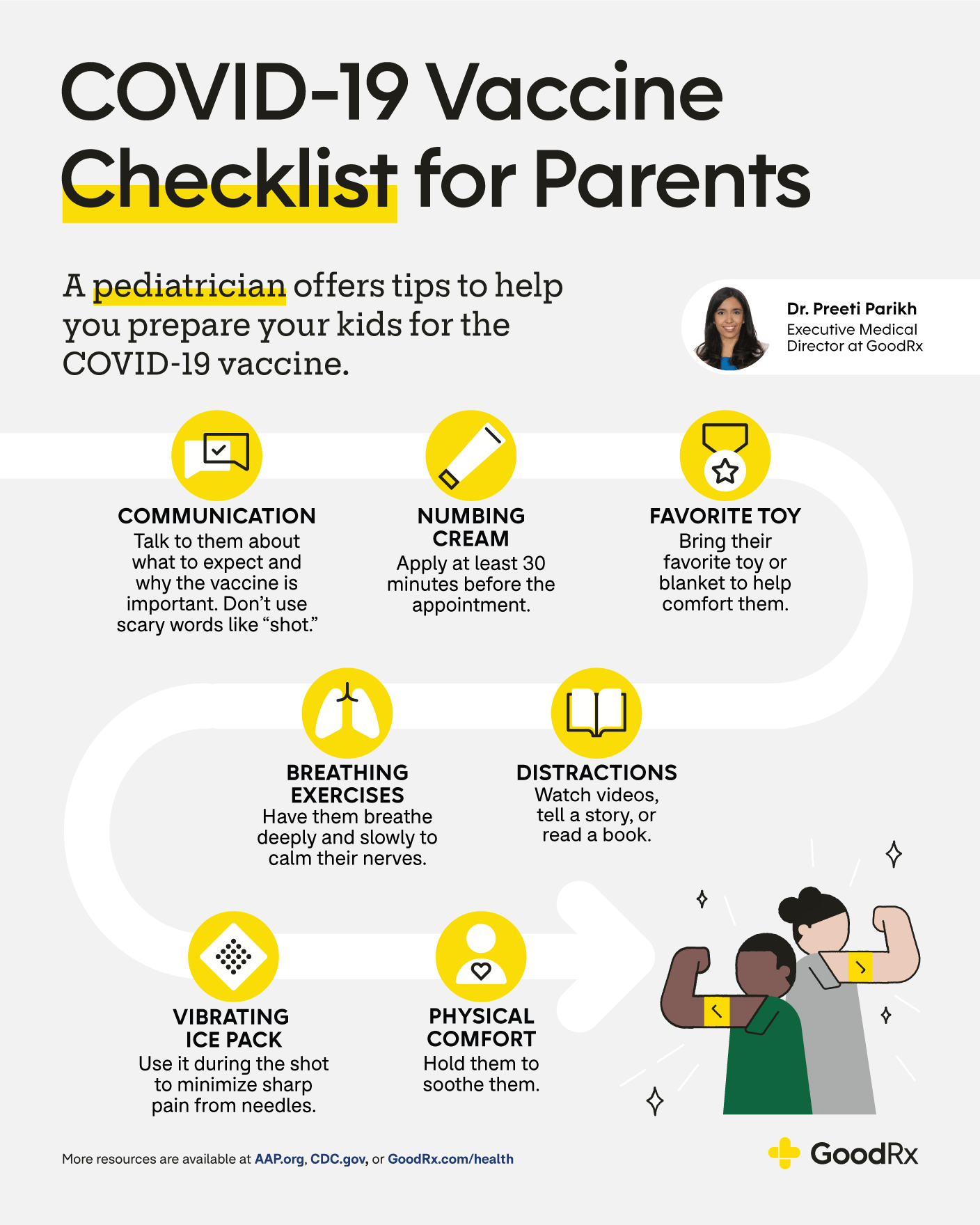
Live Updates Covid 19 In Kids Goodrx

American Academy Of Pediatrics Immunization Pages

Covid Updates C D C Recommends 2 Covid Vaccines For Very Young Children The New York Times
Aap Advises Children And Teens Age 12 And Up Get The Covid 19 Vaccine Healthychildren Org

Pediatricians And Family Physicians Toolkit Wecandothis Hhs Gov

American Academy Of Pediatrics The Aap Strongly Recommends In Person Learning For The 2021 2022 School Year And Urges All Who Are Eligible To Be Vaccinated To Protect Against Covid 19 Read More Here
_Page_1.jpg)

